Science
Why Chronic Wounds?
Wound healing, an extraordinary and complex process, is one that most of us take for granted in our daily lives.
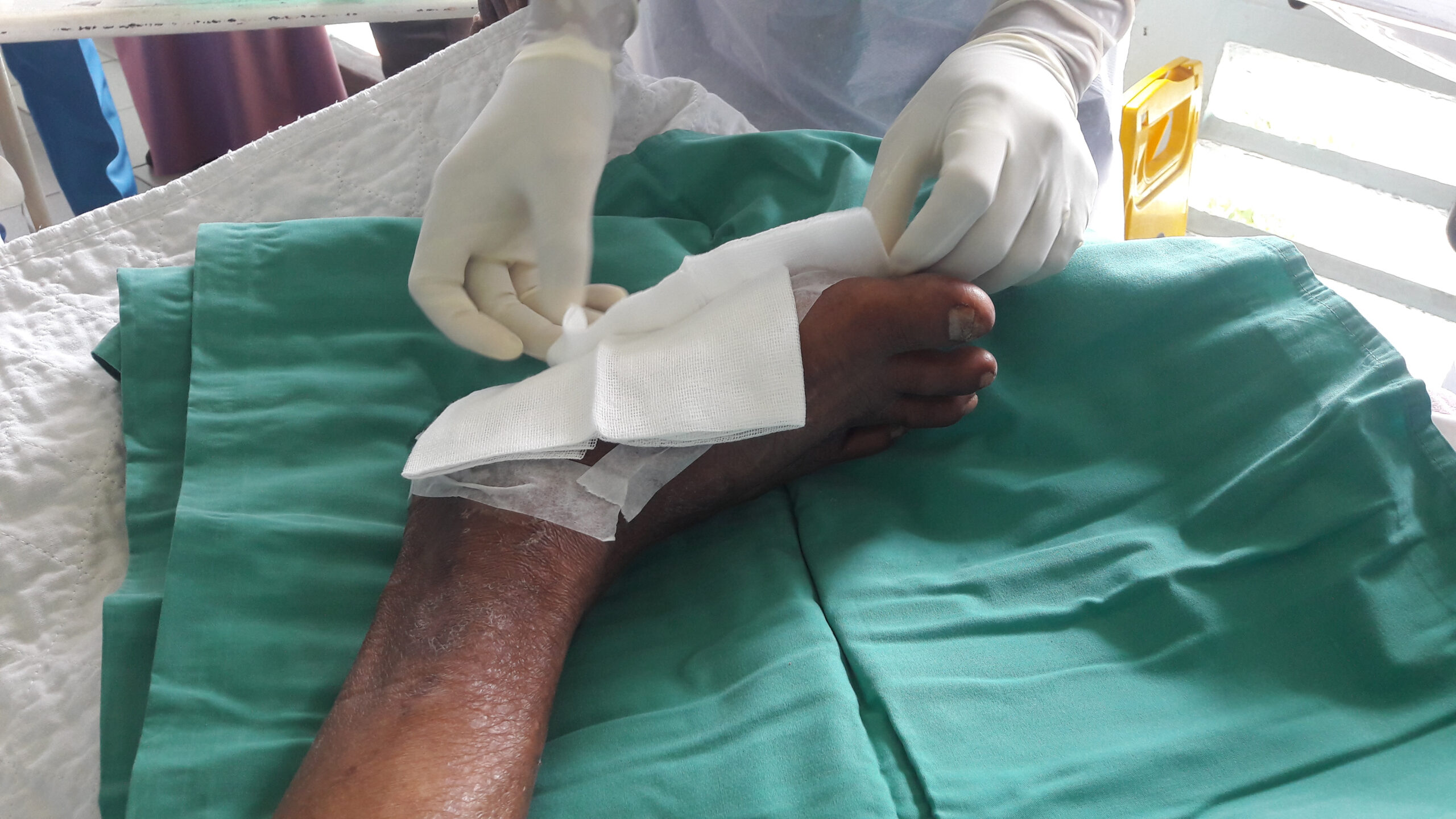
However, for patients with underlying health conditions such as diabetes & venous insufficiency, wounds can heal very slowly or not at all. In this case the wounds are considered to be chronic, often lasting months or even years. These wounds may contain slough and eschar, and exude highly. They can be extremely painful, malodorous, prone to infection and can end in amputation.
Alongside treating the patient’s underlying condition(s), the first step is to return these wounds to a healing trajectory by preparing the wound bed.
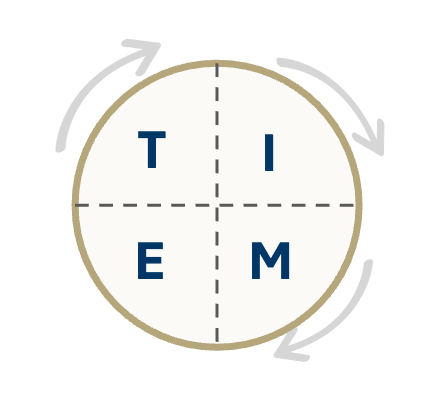
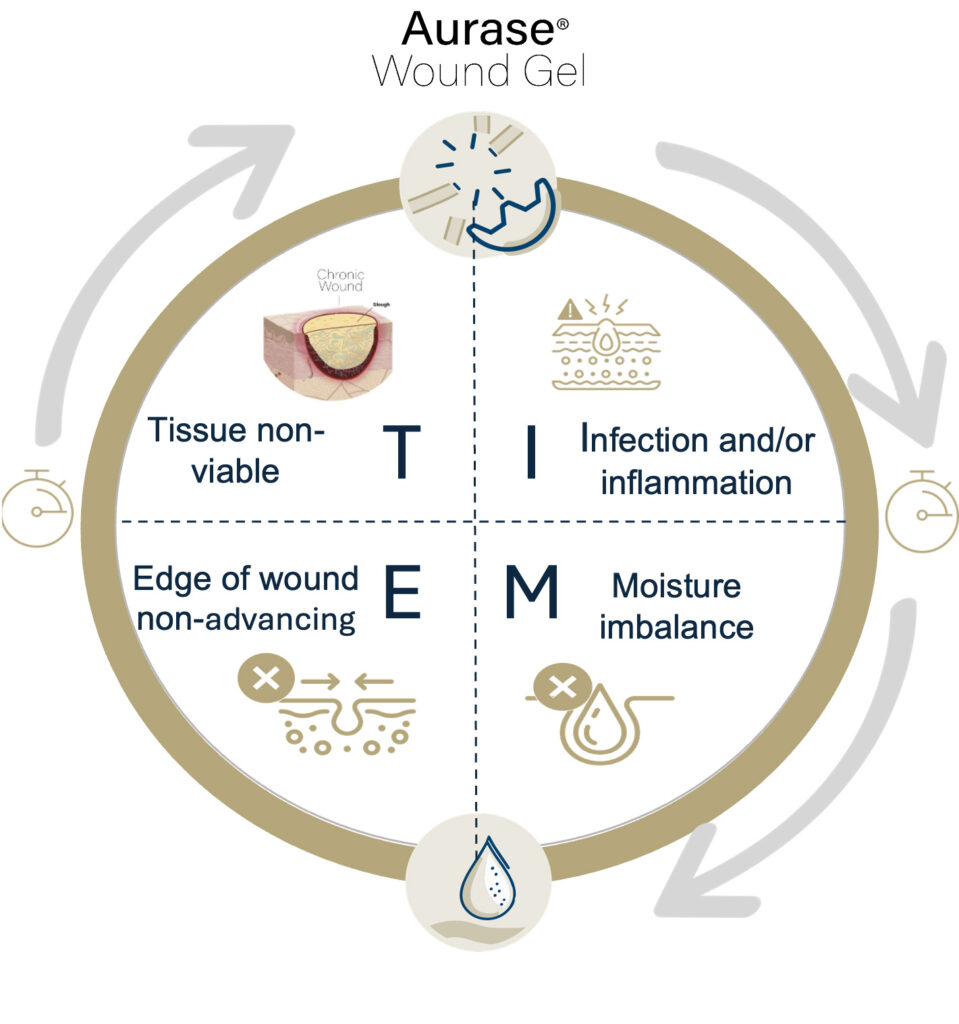
Effective wound bed preparation, a prerequisite for a chronic wound to heal, consists of four key elements:
T: The removal of non-viable tissue (debridement)
I: Management of infection and inflammation
M: Appropriate moisture balance, including management of exudate
E: Edge of wound non-advancing
These elements come together in the well-known “TIME” paradigm (the most commonly used clinical decision support tool in chronic wounds).
Currently, the physicians and nurses at the front line of treating these wounds do not have a consistent standard of care for effective wound-bed preparation that addresses all of the fundamental elements of TIME in a single treatment. Instead, there is a huge range of treatment choices available at different stages of the wound care journey, leading to complexity, inconsistency and poor treatment outcomes.
In particular, debridement is hard to address using treatments available today. Existing methods are either safe, but slow and ineffective (such as hydrogels), or rapid but resource-intensive and/or painful (such as surgery and waterjets).
There is an acute need for a single, simple, safe and easy-to-use wound care product that effectively addresses all key aspects of wound bed preparation, and fits within current standards of care and dressing change frequency.

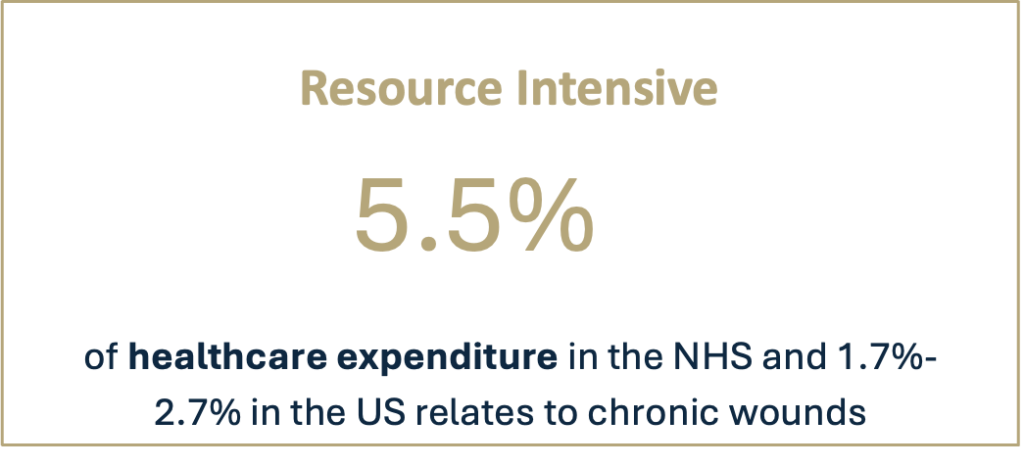
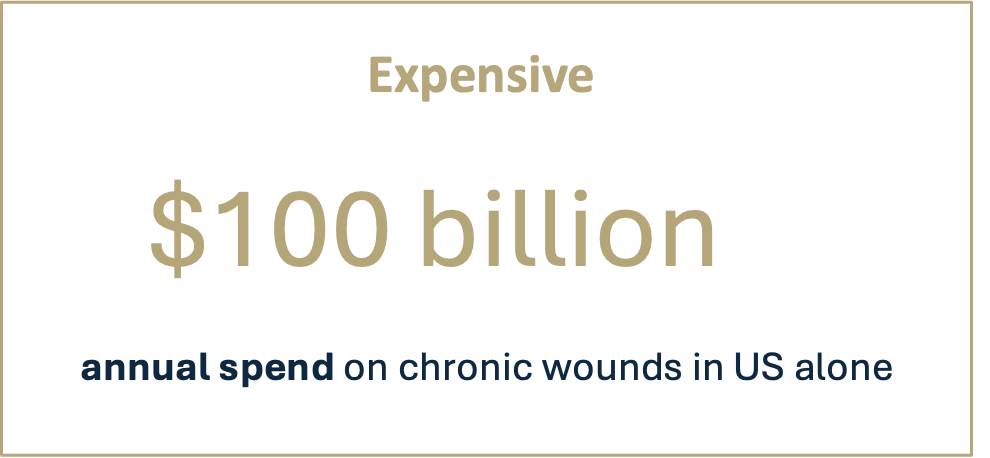
Debridement & wound bed preparation remain an unmet need in the face of rising prevalence and pressured health systems
- Industry reluctance towards drug innovation
- Patient reach & access is hard, with majority of patients not seen in hospitals
- Nurses don’t have effective community treatment options
SolasCure’s mission is to overcome these challenges to innovate wound bed preparation
Aurase Wound Gel, SolasCure’s first investigational product, is a single, effective and easy-to-use solution with strong IP, for all elements of chronic wound care.

Aims to target all elements of TIME wound management paradigm
For all stages of the wound-healing journey

Pain free & not adding to patients’ pain burden
High compatibility with established wound care products

Ease-of-use in clinics & homes
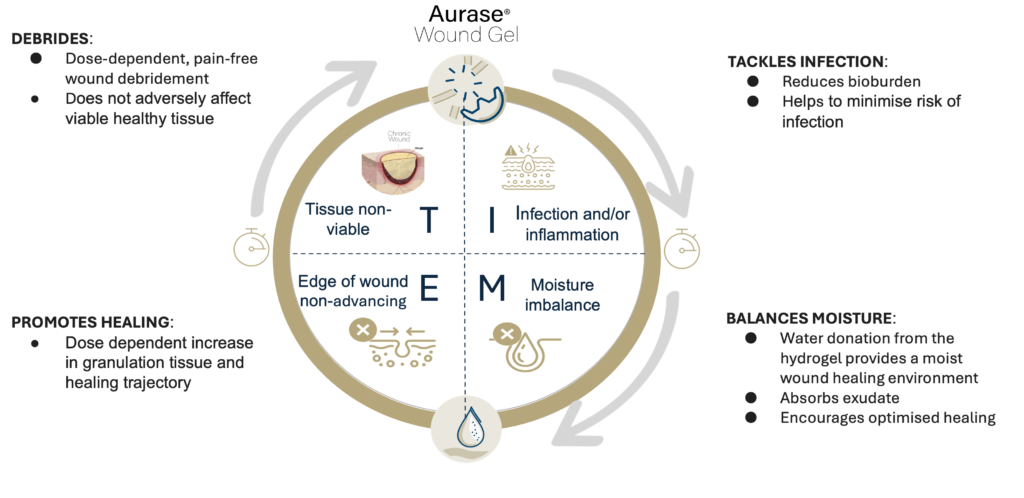
AURASE WOUND GEL ADDRESSES ALL FUNDAMENTALS OF TIME, WHICH TOGETHER ARE CRITICALLY IMPORTANT TO HEALING
- DEBRIDES:
- Dose-dependent, pain-free wound debridement
- Does not adversely affect viable healthy tissue
- PROMOTES HEALING:
- Dose dependent icrease in granulation tissue and healing trajectory
- TACKLES INFECTION:
- Reduces bioburden
- Helps to minimise risk of infection
- BALANCES MOISTURE:
- Water donation from the hydrogel provides a moist wound healing environment
- Absorbs exudate
- Encourages optmised healing
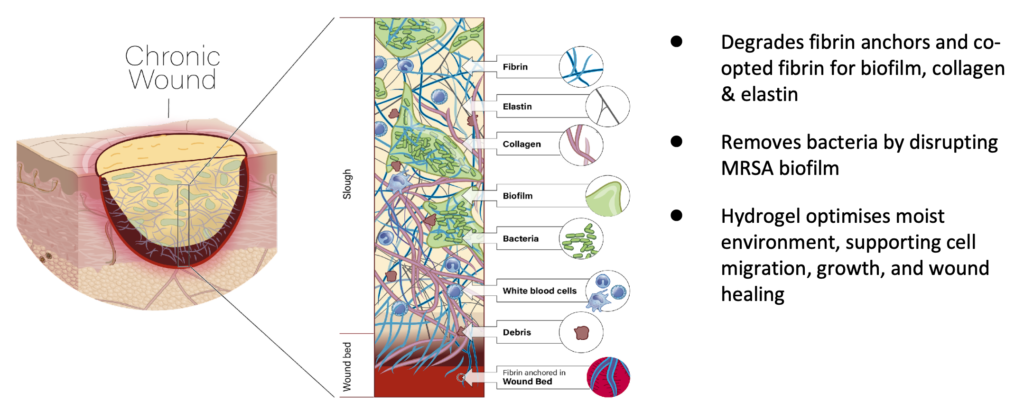
Aurase Wound Gel consists of tarumase, a highly-specific fibrinolytic enzyme isolated & cloned from maggot saliva, combined with a hydrogel for easy application on the wound. The product aims to facilitate debridement, reduce bacterial biofilm, and promote wound bed preparation and healing.
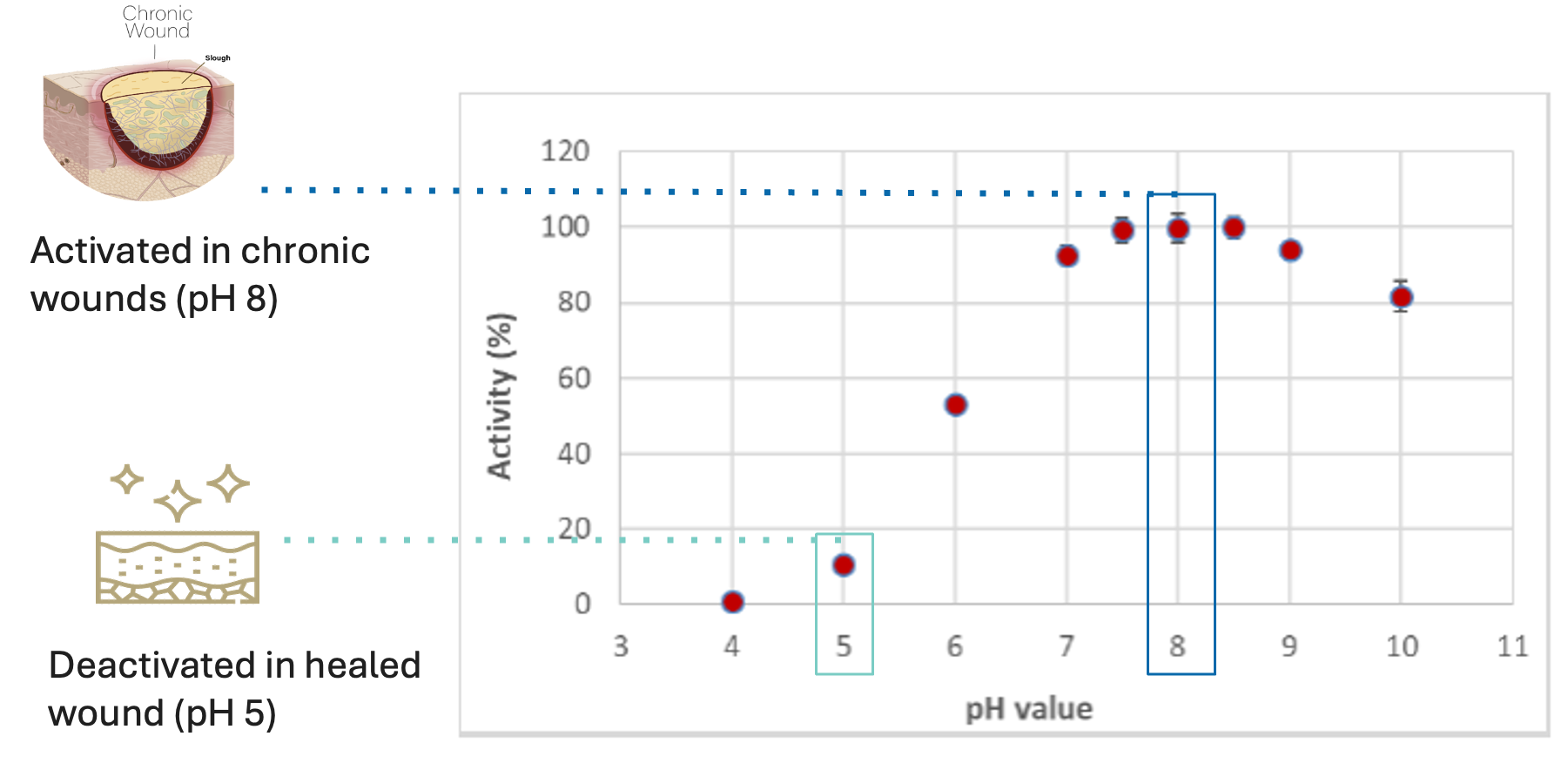
Tarumase, perfectly optimised by evolution for wound bed preparation, is a highly effective molecule for treating chronic wounds that does not affect healthy skin:
- Effective wound bed preparation
- Highly selective for fibrin present in wound slough and biofilm
- Also acts on collagen and elastin
- Supports regeneration of granulation tissue & aids healing
- No pain
- Not immunogenic
Aurase Wound Gel is a medical product under investigation. It is not available for sale or commercial distribution anywhere in the world.

Proof-of-Concept
✓ Tarumase successfully debrides wounds faster
✓ More complete debridement & improved healing at increased enzyme concentrations

Strong Safety Profile
✓ No indications of systemic absorption
✓ No antibody generation
✓ No systemic effects on coagulation

Pain-free
✓ Does not add to patients’ already existing pain burden
✓ No evidence of local tolerability issues
Exploring wound bed preparation/wound debridement in chronic ulcers, our first trial targets venous leg ulcers.

[1] Chandan K. Sen.Advances in Wound Care.Feb 2019.39-48. http://doi.org/10.1089/wound.2019.0946
[2] Fleischmann W, Grassberger M, Sherman R. Maggot Therapy: A Handbook of Maggot-Assisted Wound Healing. New York: Thieme;2004.
[3] Chernin E. Surgical maggots. South Med J. 1986;79:1143-5.
[4] Whitaker IS, Twine C, Whitaker MJ, Welck M, Brown CS, Shandall A. Larval therapy from antiquity to the present day: mechanisms of action, clinical applications, and future potential. Postgrad Med J. 2007;83(980):409-13.
[5] Fairlamb DM, Szepeshazi K, Goldsmith D, Danos P, Lev-Tov H, Young N, Hanft J, Zelen C. First clinical evaluation of the safety and efficacy of tarumase for the debridement of venous leg ulcers. Int Wound J. 2024 Mar;21(3):e14805. doi: 10.1111/iwj.14805. PMID: 38385795; PMCID: PMC10883251.




